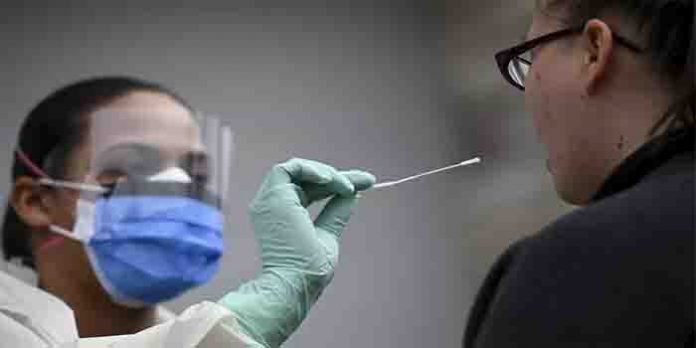
The US Food and Drug Administration has authorized the first rapid coronavirus test that can be taken at home and deliver results within minutes. The nasal swab test, which is eligible for use by patients aged 14 and over suspected of having the disease, can provide results within 30 minutes. The single-use test kit is considered more accurate than antigen tests and it is eligible to use in hospitals too. It will cost less than $50, according to the company’s website. The test kit created by California-based Lucira Health received emergency use authorization late Tuesday. The test works by swirling the self-collected sample swab in a vial that is then placed in the test unit. In 30 minutes or less, the results can be read directly from the test unit’s light-up display that shows whether a person is positive or negative for the SARS-CoV-2 virus. Positive results indicate the presence of SARS-CoV-2. Individuals with positive results should self-isolate and seek additional care from their health care provider.
© TheKarmaNews 2020, Powered by B4creations.